発表論文など
2025年
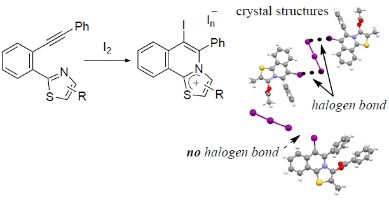
Crystal Structure of Iodinated Mesoionic
Triphenylthiazol-3-ium-4-olates: Combination of Stacking and Halogen Bonding
Interactions
Shoji Matsumoto, Shun Suzuki, Motohiro
Akazome, ChemPlusChem, 90 (11), e70052
(2025).
https://doi.org/10.1002/cplu.202500477


(↑Front coverに選ばれました)
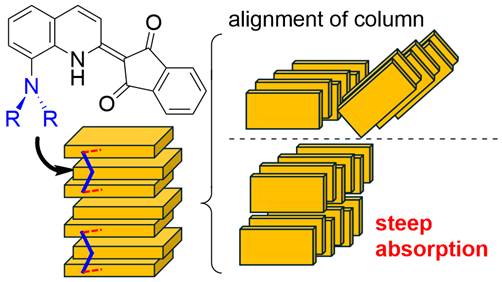
Crystal Structure of Aminoquinophthalones
and Relation to Steepness of the Absorption in the Solid State
Shoji Matsumoto, Reo Hasegawa, Motohiro
Akazome, Joji Mikami, Hiroyuki Yanai, Dyes Pigm.,
235, 112590 (2025).
https://doi.org/10.1016/j.dyepig.2024.112590
2024年
Reduction of Styrene Compounds by Hydrogen Iodide
Shoji Matsumoto, Yusuke Fukaya, Motohiro
Akazome, Tatsuo Kaiho, Synth. Commun., 54 (14), 1159-1167 (2024).
https://doi.org/10.1080/00397911.2024.2375547

Physical Separations: Chiral Discrimination of
Enantiomer by Diastereomeric Complexation With Chiral Host Compounds,
Motohiro Akazome,Shoji Matsumoto, In Comprehensive Chirality, 2nd
edition; Janine Cossy, Ed., Elsevier: Academic
Press, 2024;
Volume 10, pp 64–90. (Hardback ISBN:
978-0-323-90644-9, eBook ISBN: 9780323906456)
https://doi.org/10.1016/B978-0-32-390644-9.00006-8
JK反応の魅力 三置換オレフィン化反応の進歩,
赤染元浩,化学, 79 (5),
68-69 (2024).
2023年
Shoji Matsumoto, Makoto Takamori, Motohiro Akazzome, Molecules, 28 (7), 2896 (2023).
https://doi.org/10.3390/molecules28072896
Successful Identification of a Novel Therapeutic
Compound for Hepatocellular Carcinoma through Screening of ADAM9 Inhibitors,
Keita Ogawa, Tetsuhiro Chiba, Masato Nakamura, Jun Arai, Jiaqi Zhang, Yaojia Ma, Na Qiang, Junjie Ao, Sae Yumita, Takamasa Ishino, Motoyasu Kan, Terunao Iwanaga, Miyuki Nakagawa, Kisako Fujiwara, Takafumi Sakuma, Hiroaki Kanzaki, Keisuke Koroki, Yuko Kusakabe, Kazufumi Kobayashi, Naoya Kanogawa, Soichiro Kiyono, Takayuki Kondo, Ryo Nakagawa, Sadahisa Ogasawara, Ryosuke Muroyama, Shingo Nakamoto, Tatsuo Kanda, Hitoshi Maruyama, Jun Kato, Shoji Matsumoto, Takayoshi Arai, Shinichiro Motohashi Naoya Kato, Anticancer Research, 43 (3), 1043-1052 (2023).
※当研究室で合成された化合物が活性評価されました。
2022年
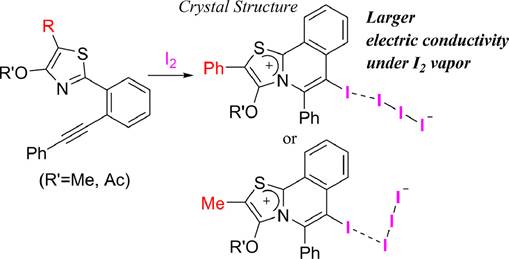
Relationship between Halogen-halogen
Interaction and Electric Conductivity in Thiazolo[2,3-a]isoquinolin-7-ium
Triiodides,
Shoji Matsumoto, Ryuta Sumida, Motohiro Akazzome, J. Mol. Struct., 1264, 133306 (2022).
https://doi.org/10.1016/j.molstruc.2022.133306

2021年
Chemoselective Reduction of α,β-Unsaturated
Carbonyl and Carboxylic Compounds by Hydrogen Iodide,
Shoji Matsumoto, Hayato Marumoto, Motohiro Akazzome, Yasuhiko Otani, Tatsuo Kaiho, Bull. Chem. Soc. Jpn., 94 (2), 590-599 (2021).
https://doi.org/10.1246/bcsj.20200341
CCL299, a
Benzimidazole Derivative, Induces G1 Phase Arrest and Apoptosis in Cancer Cells,
Yoshifumi Ohno, Ruipong Yi, Akiko Suganami, Yutaka Tamura, Akio Matsumoto, Shoji Matsumoto, Kengo Saito, Hiroshi Shirasawa, Anticancer Research, 41 (2), 699-706 (2021).
https://doi.org/10.21873/anticanres.14821
※当研究室で合成された化合物が活性評価されました。
2020年
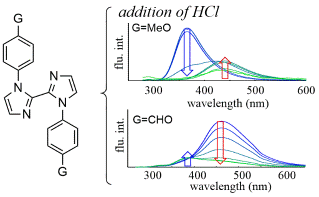
Change in the Fluorescence of
1,1′-Diaryl-2,2′-biimidazoles upon the Addition of Acid,
Shoji Matsumoto, Seigi Tachibana, Motohiro Akazome, Heterocycles, 100 (10), 1666-1677 (2020).
https://doi.org/10.3987/COM-20-14309
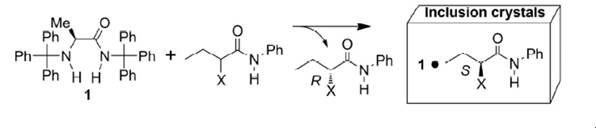
Molecular
Recognition by Inclusion Crystals of Chiral Host Molecules Having Trityl and
Related Bulky Groups,
Motohiro
Akazome, Shoji Matsumoto, In Advances in Organic Crystal Chemistry,
Comprehensive Reviews 2020, M. Sakamoto, H. Uekusa (Eds.): Springer, Tokyo,
2020,
pp. 457-475.
(ISBN 978-981-15-5084-3)

2019年
Selective Synthesis and
Optical Properties of Diimidazo[1,2-a:5′,1′-c]quinoxaline Derivatives,
Shoji Matsumoto, Shunsuke Abe, Motohiro
Akazome, Tetrahedron, 75 (26), 3657-3665 (2019).
https://doi.org/10.1016/j.tet.2019.05.041
乙種1・2・3・5・6類危険物取扱者試験テキスト&問題集 第2版,
赤染元浩監修,ナツメ社,2019年3月1日発行 ISBN978-4-8163-6598-0.

2018年
Synthesis of Iodinated Thiazolo[2,3-a]isoquinolinium
Salts and Their Crystal Strucutres with/without
Halogen Bond,
Shoji Matsumoto, Ryuta Sumida, Sia Er Tan,
and Motohiro Akazome, Heterocycles, 97 (2),
755-775 (2018).
https://doi.org/10.3987/COM-18-S(T)47

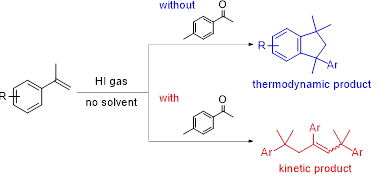
Selective Formation
of Internal Olefinic Trimer of α‑Methylstyrenes with HI Gas and Ketones,
Shoji Matsumoto, Takehisa Oseki, Motohiro Akazome, and Yasuhiko Otani, ACS Omega, 3 (12),
17928-17935 (2018).
https://doi.org/10.1021/acsomega.8b03168
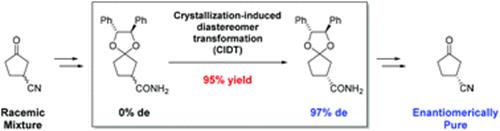
Preparation of
Chiral 3-Oxocycloalkanecarbonitrile and Its Derivatives by
Crystallization-Induced Diastereomer Transformation of Ketals with Chiral
1,2-Diphenylethane-1,2-diol,
Yohei Yamashita, Daisuke Maki, Shiho Sakurai, Takumi
Fuse, Shoji Matsumoto, Motohiro Akazome, RSC Adv.,
8 (57), 32601-32609 (2018).
https://doi.org/10.1039/c8ra06611f

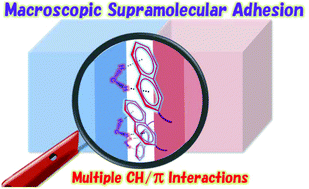
Exploiting CH/π Interactions
in Robust Supramolecular Adhesives,
Taiki Yamate, Takayuki Fujiwara, Toru Yamaguchi,
Hiroshi Suzuki, Motohiro Akazome, Polym. Chem., 9
(32), 4303-4308 (2018).
https://doi.org/10.1039/c8py00592c


(↑Inside front coverに選ばれました)
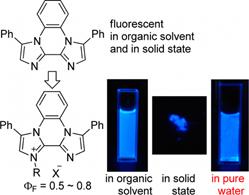
Incrementing
Stokes Shifts through the Formation of 2,2′-Biimidazoldiium Salts,
Shoji Matsumoto, Mei Watanabe, Motohiro
Akazome, Org. Lett., 20 (12),
3613-3617 (2018).
https://doi.org/10.1021/acs.orglett.8b01376

Polyacrylamide-Scaffold
Adhesive Bearing Multiple Benzene Rings Forming CH/π Interactions with
Polyolefin,
Taiki Yamate, Hiroshi Suzuki, Takayuki Fujiwara, Toru
Yamaguchi, Motohiro Akazome, Adv. Mater. Lett., 9
(7), 526-530 (2018).
https://doi.org/10.5185/amlett.2018.2055
2017年
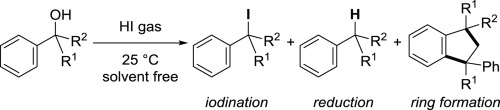
Selective Reaction of Benzyl
Alcohols with HI Gas: Iodination, Reduction, and Indane Ring Formations,
Shoji Matsumoto, Masafumi Naito, Takehisa Oseki, Motohiro Akazome, Yasuhiko Otani, Tetrahedron, 73 (52),
7254-7259 (2017).
https://doi.org/10.1016/j.tet.2017.11.009

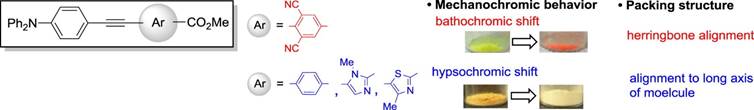
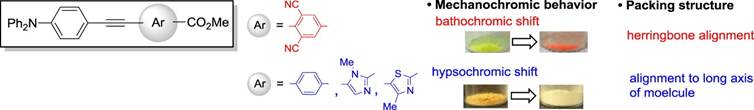
Relationship between Mechanochromic Behavior and Crystal Structures in
Donor-π-acceptor Compounds Consisted of Aromatic Rings with Ester Moiety as an
Acceptor,
Shoji Matsumoto, Jun Moteki, Yuji Ito, Motohiro Akazome, Tetrahedron
Lett., 58 (36), 3512-3516
(2017).
https://doi.org/10.1016/j.tetlet.2017.07.085
Effective
Design of Supramolecular Polymer Adhesives Based on Multiple CH/π interactions,
Taiki Yamate, Hiroshi Suzuki, Kazuhisa Kumazawa, Takayuki Fujiwara, Toru Yamaguchi, Motohiro Akazome, Mol. Sys. Des. Eng., 2 (2), 214-222 (2017).
https://doi.org/10.1039/c7me00022g
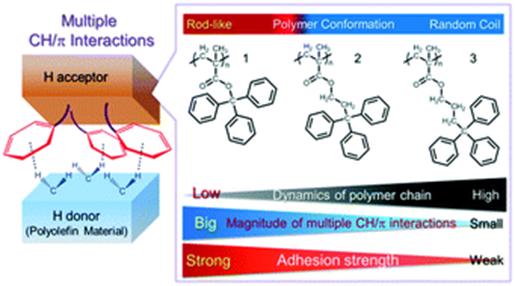
(↑Back coverに選ばれました)
完全攻略!ここが出る!毒物劇物取扱者試験テキスト&問題集
赤染元浩監修,ナツメ社,2017年8月1日発行 ISBN978-4-8163-6277-4.

A Practical and Scalable Synthesis of a Glucokinase Activator via Diastereomeric Resolution and Palladium-Catalyzed C–N Coupling Reaction,
Yohei Yamashita, Yasuhiro Morinaga, Makoto Kasai,
Takao Hashimoto, Yuji Takahama, Atsushi Ohigashi, Satoshi Yonishi, Motohiro Akazome,
Org. Process Res. Dev., 21 (3),
346-356 (2017).
https://doi.org/10.1021/acs.oprd.6b00415
2016年
一発合格!甲種危険物取扱者試験<ここが出る>問題集
赤染元浩監修,ナツメ社,2016年8月1日発行 ISBN978-4-8163-6061-9.
CH/π Interactions for
Macroscopic Interfacial Adhesion Design,
Taiki Yamate, Kazuhisa Kumazawa, Hiroshi Suzuki, Motohiro Akazome, ACS
Macro Lett., 5 (7), 858-861
(2016).
https://doi.org/10.1021/acsmacrolett.6b00265

Formation of Benzimidazoisoquinolinium and Benzimidazoisoindolinum
Cyclic Systems by the Reaction of 2-(2-Alkynylphenyl)benzimidazoles with Iodine
and Iodine–iodine Interaction Including
Halogen Bonding in Their Crystal Structures,
Shoji Matsumoto, Shu Kikuchi,
Naoto Norita, Hyuma Masu, Motohiro
Akazome, J. Org. Chem., 81 (13), 5322-5329
(2016).
https://doi.org/10.1021/acs.joc.6b00607
スパイラル有機化学−基礎から応用、発展へ!−
赤染元浩,河内 敦,松本祥治,三野 孝 著,筑波出版会,2016年4月11日発行 ISBN978-4-924753-61-7

2015年
一発合格!乙種第4類危険物取扱者試験<ここが出る>問題集
赤染元浩監修,ナツメ社,2015年10月1日発行 ISBN978-4-8163-5902-6.

Chiral Recognition by
Inclusion Crystals of Amino-Acid Derivatives Having Trityl Groups,
Motohiro Akazome, In Advances
in Organic Crystal Chemistry, Comprehensive Reviews 2015, R. Tamura, M.
Miyata (Eds.): Springer, Tokyo, 2015,
pp. 463-482. (ISBN 978-4-431-55554-4)

HI Gas as a Reagent
for α-Alkylation Reaction with Two Ketone Molecules,
Shoji Matsumoto, Seigo Koitabashi, Yasuhiko Otani, Motohiro
Akazome, Tetrahedron
Lett., 56 (29), 4320-4323 (2015).
https://doi.org/10.1016/j.tetlet.2015.05.071
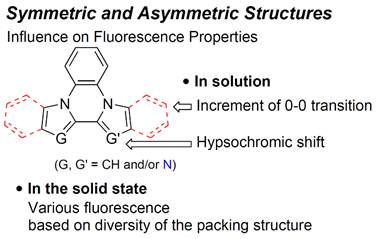
Systematic
Investigation of Fluorescence Properties of Symmetric and Asymmetric Diazolo[1,2-a:2′,1′-c]quinoxaline Derivatives,
Shoji Matsumoto,
Keisuke Sakamoto, Motohiro Akazome, Heterocycles, 91 (4), 795-814
(2015).
https://doi.org/10.3987/COM-15-13189
2014年
一発合格!乙種第4類危険物取扱者試験テキスト&問題集
赤染元浩監修,ナツメ社,2014年12月21日発行 ISBN978-4-8163-5761-9.

多様な状態で蛍光発光可能な縮環型有機化合物群の開発,
松本祥治,Jasco Report, 56 (1), 15-22 (2014).
Binding of Acetylcholine and Quaternary
Ammonium Compounds to a Cs-Symmetric
Bowl-Shaped Tripeptide of 2-(3-Aminophenyoxy)propanoic Acids Acting as a Ditopic Receptor,
Motohiro Akazome, Norihiro Hamada, Koji Takagi, Daisuke Yagyu, Shoji
Matsumoto, Tetrahedron Lett., 55
(14), 2226-2229 (2014).
https://doi.org/10.1016/j.tetlet.2014.02.066

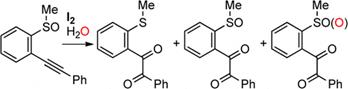
Formation of Benzo[c]thiophene-1-aminium Iodide by the Reaction of o-Alkynylbenzothioamide
with Iodine,
Shoji Matsumoto,
Daiki Takada, Hirokazu Kageyama, Motohiro Akazome, Tetrahedron Lett., 55 (5),
1082-1085 (2014).
https://doi.org/10.1016/j.tetlet.2013.12.094
Synthesis and Optical Properties of 2,2'-Biimidazole and Benzo[d]imidazole Derivatives: Changing π-Conjugation by Photoexcitation,
Shoji Matsumoto, Yu Zhao, Motohiro
Akazome, Heterocycles, 88 (1), 261-273 (2014).
https://doi.org/10.3987/COM-13-S(S)12

さらに過去の論文などはこちらから。