◆最近の研究業績
2025年
-
Asymmetric synthesis of β-amino cyanoesters with contiguous tetrasubstituted carbon centers by halogen-bonding catalysis with chiral halonium salt, Yasushi Yoshida*, Maho Aono, Takashi Mino and Masami Sakamoto, Beilstein J. Org. Chem., 2025, 21, 547-555.
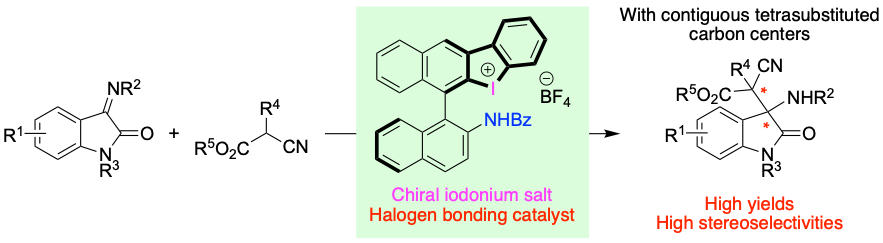
-
Pd-Catalyzed asymmetric allylic amination with isatin using a P,olefin-type chiral ligand with C–N bond axial chirality, Natsume Akimoto, Kaho Takaya, Yoshio Kasashima, Kohei Watanabe, Yasushi Yoshida and Takashi Mino, Beilstein J. Org. Chem., 2025, 21, 1018-1023.
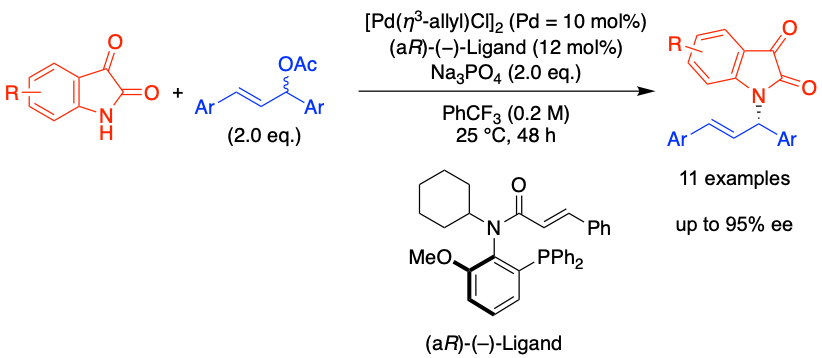
2024年
-
Synthesis of Branch-Type 3 Allylindoles from N Alkyl N cinnamyl-2-ethynylaniline Derivatives Using π–Allylpalladium Chloride Complex as a Catalyst, Kohei Watanabe, Keita Nakano, Hayato Sato, Toshiki Yamaoka, Yasushi Yoshida, Ryo Takita, Yoshio Kasashima, Masami Sakamoto, and Takashi Mino, J. Org. Chem., 2024, 89, 8111–8119.
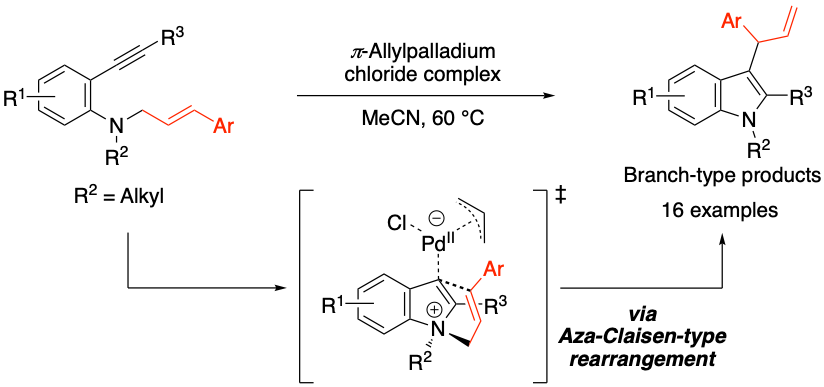
2023年
-
Axially chiral N-alkyl-N-cinnamoyl amide type P,olefin ligands for Pd-catalyzed reactions, Takashi Mino, Kaho Takaya, Kaito Koki, Natsume Akimoto, Yasushi Yoshida, Yoshio Kasashima, and Masami Sakamoto, Org. Biomol. Chem., 2023, 21, 2775-2778.
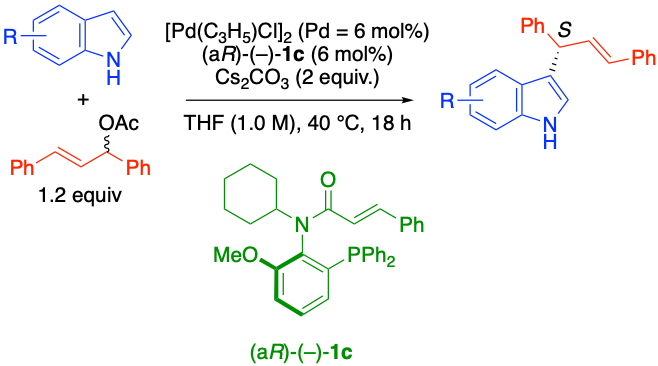
-
Chiral Bromonium Salt (Hypervalent Bromine(III)) with N-Nitrosamine as a Halogen-Bonding Bifunctional Catalyst, Yasushi Yoshida, Tatsuya Ao, Takashi Mino, Masami Sakamoto, Molecules, 2023, 28, 384.
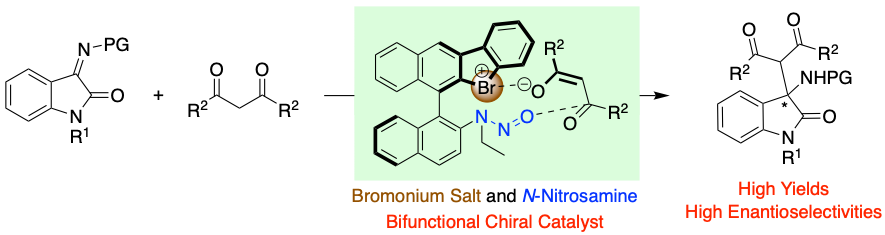
-
Intramolecular Azo Coupling Reaction of Binaphthyl Compounds: Synthesis of Pyrazole-Containing Helicene-Like Molecules, Yasushi Yoshida, Naoyuki Aso, Takashi Karatsu, Takashi Mino, and Masami Sakamoto, Org. Lett., 2023, 25, 3412–3416.
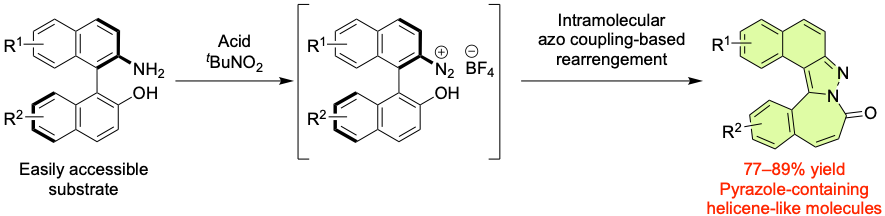
-
Formal [3 + 2] Cycloaddition of α-Imino Esters with Azo Compounds: Facile Construction of Pentasubstituted 1,2,4-Triazoline Skeletons, Yasushi Yoshida, Hidetoshi Ida, Takashi Mino, and Masami Sakamoto, Molecules, 2023, 28, 4339.
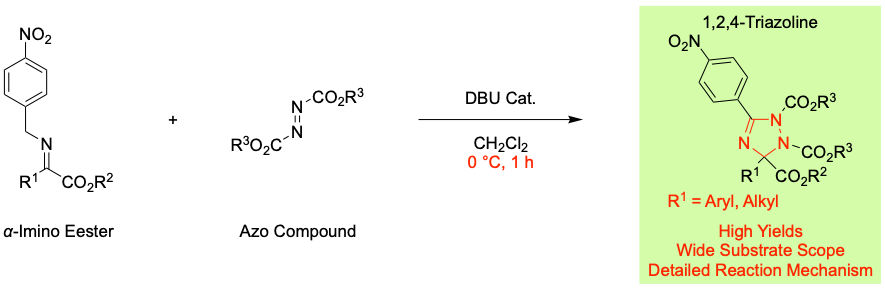
2022年
-
Attrition-Enhanced Asymmetric Transformation of Axially Chiral Nicotinamides by Dynamic Chiral Salt Formation, Takumi Nakamura, Hiroki Ishikawa, Kazuma Ban, Yasushi Yoshida, Takashi Mino, Yoshio Kasashima, Masami Sakamoto, ChemPlusChem, 2022, 87, e202100504.
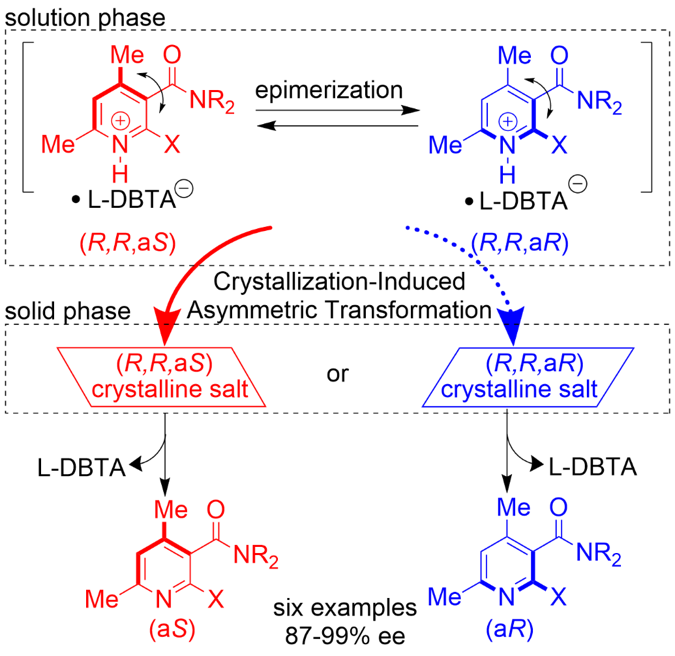
-
Chiral Symmetry Breaking of Monoacylated Anhydroerythritols and meso-1,2-Diols via Crystallization-Induced Deracemization, Kazutaka Sanada, Aoi Washio, Hiroki Ishikawa, Yasushi Yoshida, Takashi Mino, Masami Sakamoto, Angew. Chem. Int. Ed., 2022, 61, e202201268.
Press Release
(https://www.chiba-u.ac.jp/general/publicity/press/files/2021/20220310_1.pdf)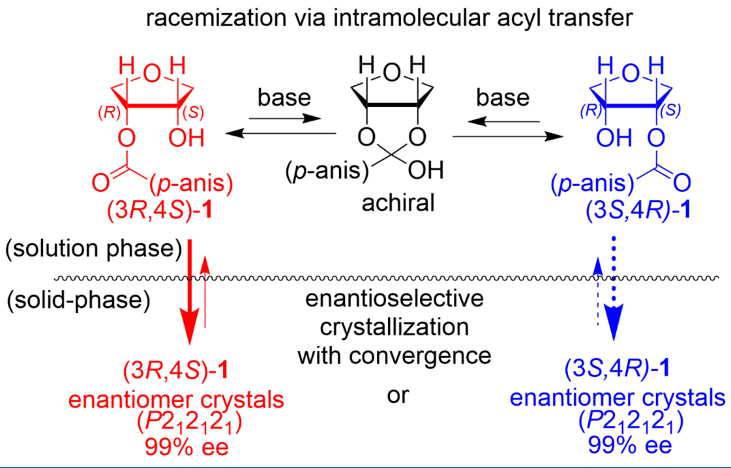
-
Behavior of All Chiral Standard Amino Acids for Chiral Symmetry Breaking of p-Anisoin, Norika Miyazaki, Kazutaka Sanada, Takumi Nakamura, Aoi Washio, Yasushi Yoshida, Takashi Mino, Yoshio Kasashima, Masami Sakamoto, Cryst. Growth Des. 2022, in press. doi; 10.1021/acs.cgd.2c00480.
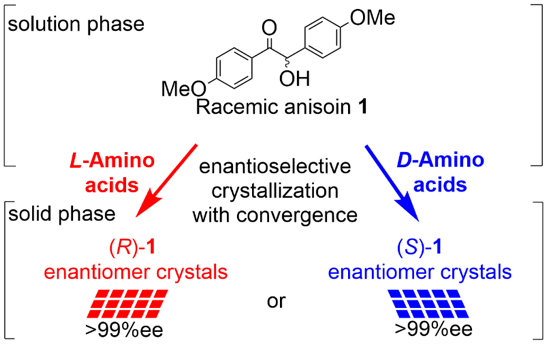
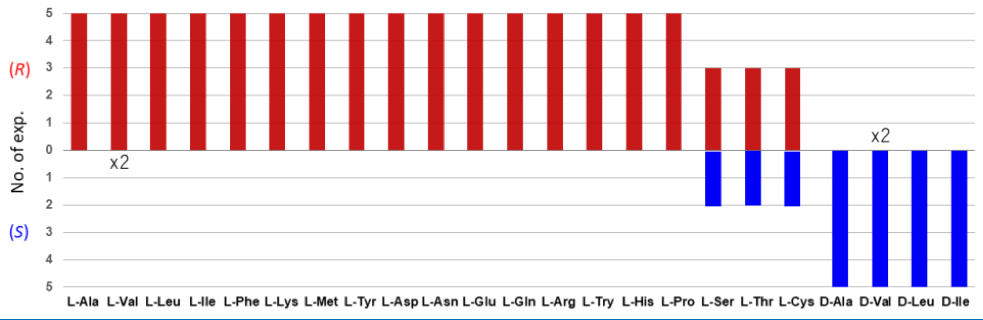
-
Synthesis of 3-Allylindoles via Annulation of N-Allyl-2-ethynylanilineDerivatives Using a P,Olefin Type Ligand/Pd(0) Catalyst, Takashi Mino, Toshiki Yamaoka, Kohei Watanabe, Chihiro Masuda, Shohei Kasano, Yasushi Yoshida, Ryo Takita, Yoshio Kasashima, Masami Sakamoto, J. Org. Chem., 2022, 87, 7365–7377.
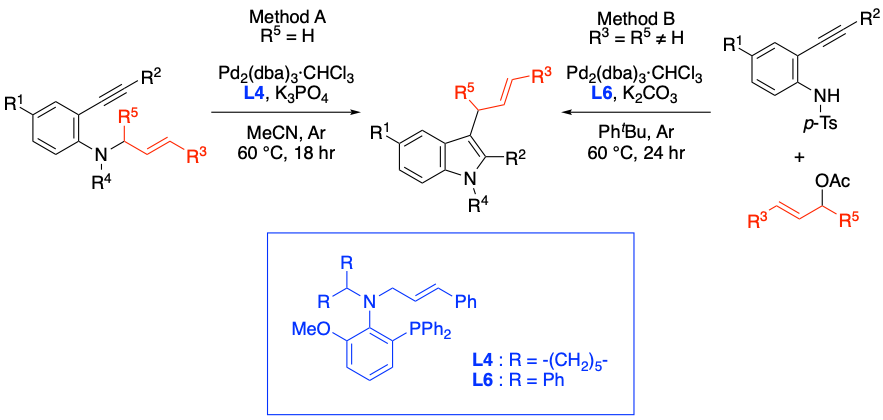
-
Chiral Binaphthyl-Based Iodonium Salt (Hypervalent Iodine(III)) as Hydrogen- and Halogen-Bonding Bifunctional Catalyst: Insight into Abnormal Counteranion Effect and Asymmetric Synthesis of N,S-Acetals, Yasushi Yoshida, Tappei Fujimura, Takashi Mino, Masami Sakamoto, Adv. Synth. Catal., 2022, 364, 1091–1098.
Selected as a front cover picture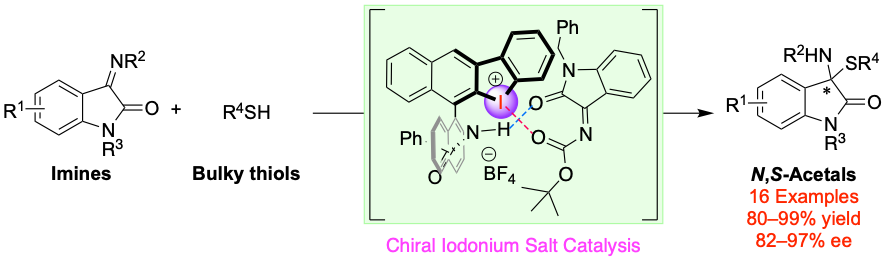
2021年
-
Chirogenesis and Amplification of Molecular Chirality Using Optical Vortices, Masami Sakamoto, Naohiro Uemura, Rei Saito, Haruna Shimobayashi, Yasushi Yoshida, Takashi Mino, Takashige Omatsu, Angew. Chem. Int. Ed., 2021, 60, 12819–12823.
Press Release
(https://www.chiba-u.ac.jp/general/publicity/press/files/2021/20210416Chirality.pdf)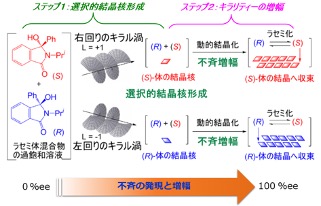
-
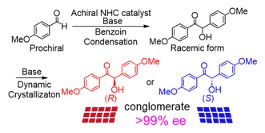
Asymmetric Anisoin Synthesis Involving Benzoin Condensation Followed by Deracemization, Aoi Washio, Momoka Hosaka, Yasushi Yoshida, Takashi Mino, Yoshio Kasashima, Masami Sakamoto, Cryst. Growth Des., 2021, 21, 24236–2428.
-
Asymmetric Synthesis of Isoindolinone by Irradiation of Phthalimide under deracemization conditions, Takumi Nakamura, Kazuma Ban, Yasushi Yoshida, Takashi Mino, Yoshio Kasashima, Masami Sakamoto, Chem. Eur. J., 2021,16338–16341. Hot Paper
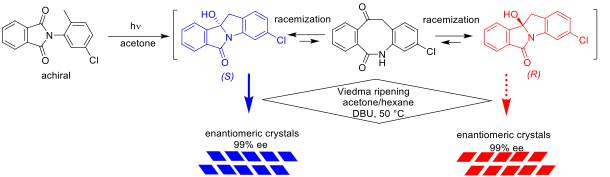
-
Chiral Symmetry Breaking of Racemic 3-Phenylsuccinimides via Crystallization-Induced Dynamic Deracemization, Kazutaka Sanada, Aoi Washio, Kazuki Nishihata, Fumitoshi Yagishita, Yasushi Yoshida, Takashi Mino, Yoshio Kasashima, Shinichi Suzuki, Masami Sakamoto, Cryst Growth Des., 2021, 21, 6051–6055.
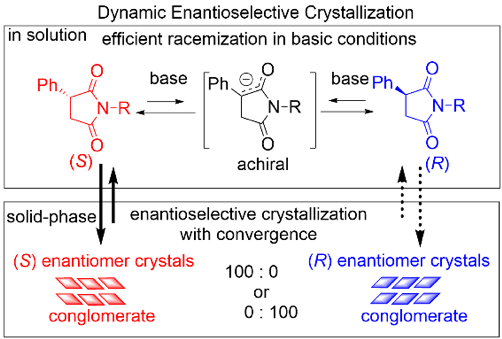
-
Chiral P,Olefin Ligands with Rotamers for Palladium-Catalyzed Asymmetric Allylic Substitution Reactions, Takashi Mino, Daiki Yamaguchi, Manami Kumada, Junpei Youda, Hironori Saito, Junya Tanaka, Yasushi Yoshida, Masami Sakamoto, Synlett, 2021, 32, 532–538.
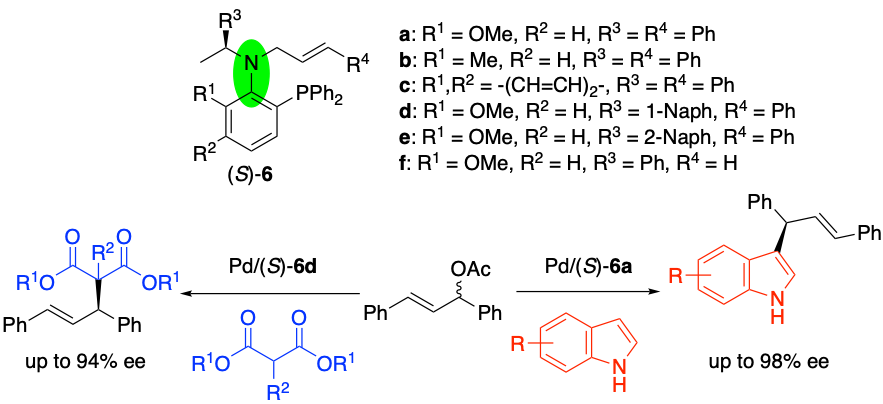
-
Cinnamoyl amide type chiral P,olefin ligands for Pd-catalyzed reactions, Takashi Mino, Yohei Fujisawa, Shizuki Yoshida, Mitsuru Hirama, Takumu Akiyama, Ryo Saito, Yasushi Yoshida, Yoshio Kasashima, Masami Sakamoto, Org. Biomol. Chem., 2021, 19, 10385-10389.
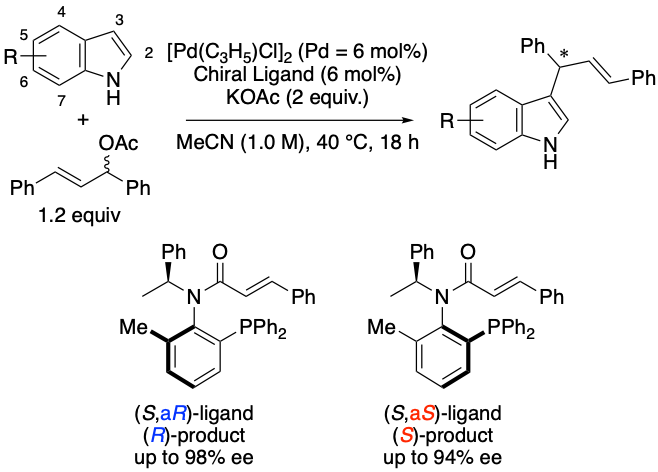
-
Phase-transfer Catalysed Asymmetric Synthesis of α-Chiral Tetrasubstituted α-Aminothioesters, Yasushi Yoshida, Reina Kasuya, Takashi Mino, Masami Sakamoto, Org. Biomol. Chem., 2021, 19, 6402–6406.
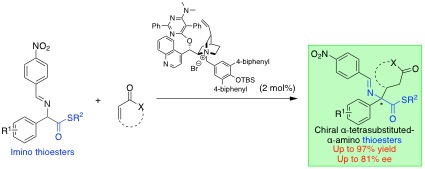
-
Iminophosphorane-mediated Regioselective Umpolung Alkylation Reaction of α-Iminoesters, Yasushi Yoshida, Mayu Kukita, Kazuki Omori, Takashi Mino, Masami Sakamoto, Org. Biomol. Chem., 2021, 19, 4551–4564.
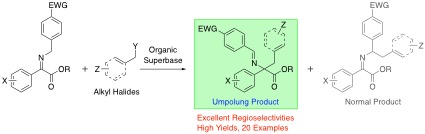
-
Bromonium Salts: Diaryl-λ3-bromanes as Halogen-bonding Organocatalysts, Yasushi Yoshida, Seitaro Ishikawa, Takashi Mino, Masami Sakamoto, Chem. Commun., 2021, 57, 2519–2522.
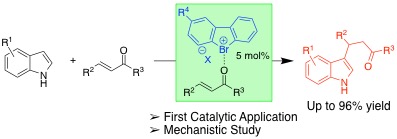
-
Chiral Hypervalent Bromine(III) (Bromonium Salt): Hydrogen- and Halogen-Bonding Bifunctional Asymmetric Catalysis by Diaryl-λ3-bromanes, Yasushi Yoshida, Takashi Mino, Masami Sakamoto, ACS Catal., 2021, 11, 13028–13033. Press Release (https://www.chiba-u.ac.jp/general/publicity/press/files/2021/20211207_1.pdf)
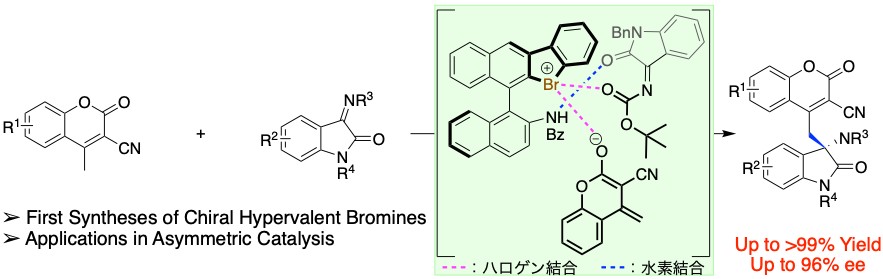
-
Influence of N-Substituents on Photovoltaic Properties of Singly Bay-Linked Dimeric Perylene Diimides, Seiichiro Izawa, Kentaro Uchida, Mayuko Nakamura, Keisuke Fujimoto, Jérémy Roudin, Ji Hyun Lee, Toshiyasu Inuzuka, Takumi Nakamura, Masami Sakamoto, Yasuo Nakayama, Masahiro Hiramoto, Masaki Takahashi, Chem. Eur. J., 2021, 27, 14081–14091.
-
5,11-Diazadibenzo[hi,qr]tetracene: Synthesis, Properties, and Reactivity toward Nucleophilic Reagents, Keisuke Fujimoto, Satoshi Takimoto, Shota Masuda, Toshiyasu Inuzuka, Kazutaka Sanada, Masami Sakamoto, Masaki Takahashi, Chem. Eur. J., 2021, 27, 8951–8955.
-
Curved Perylene Diimides Fused with Seven-Membered Rings, Keisuke Fujimoto, Seiichiro Izawa, Ayumu Takahashi, Toshiyasu Inuzuka, Kazutaka Sanada, Masami Sakamoto, Yasuo Nakayama, Masahiro Hiramoto, Masaki Takahashi, Chem Asian J., 2021, 16, 690–695.
-
<Book> Uemura, Naohiro; Toyoda, Seiya; Shimizu Waku, Yoshida, Yasushi; Mino, Takashi; Sakamoto, Masami, Symmetry, Absolute Asymmetric Synthesis Involving Chiral Symmetry Breaking in Diels-Alder Reaction, in [Symmetry] Special Issue book: Chemical Symmetry Breaking, R. Tamura Ed., MDPI, Basel • Beijing • Wuhan • Barcelona • Belgrade • Manchester • Tokyo • Cluj • Tianjin, 2020, 12, 910.
-
<Book> 坂本昌巳,結晶化誘起動的不斉増幅法を利用した光不斉反応,CSJカレントレビュー 有機光反応化学の新展開,㈱化学同人, 2021.
-
<受賞> 藤村竜平,「新規不斉触媒としてのキラルハロニウム塩の開発および応用」,優秀ポスター賞,第24回ヨウ素学会シンポジウム
-
<受賞> 中村拓海,「キラル塩形成を伴う動的晶出法を用いたニコチンアミド誘導体の軸不斉制御」,優秀ポスター賞,第29回有機結晶シンポジウム
-
<受賞> 宮﨑紀佳,「微少な不⻫源存在下でのベンゾイン縮合による不⻫の発現と増幅」,優秀ポスター発表賞,第11回CSJ化学フェスタ2021
2020年
-
Attrition-Enhanced Deracemization and Absolute Asymmetric Synthesis of Flavanones from Prochiral Precursors, Waku Shimizu, Naohiro Uemura, Yasushi Yoshida, Takashi Mino, Yoshio Kasashima, Masami Sakamoto, Cryst. Growth Des., 2020, 20, 5676–5681.
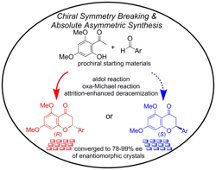
-
Chiral Symmetry Breaking of Thiohydantoins by Attrition-Enhanced Deracemization, Naohiro Uemura, Momoka Hosaka, Aoi Washio, Yasushi Yoshida, Takashi Mino, Masami Sakamoto, Cryst. Growth Des., 2020, 20, 4898–4903.
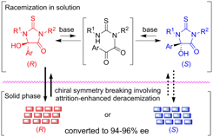
-
Absolute Asymmetric Synthesis Involving Chiral Symmetry Breaking in Diels–Alder Reaction, Naohiro Uemura, Seiya Toyoda, Waku Shimizu, Yasushi Yoshida, Takashi Mino, Masami Sakamoto, Symmetry, 2020, 12, 910.
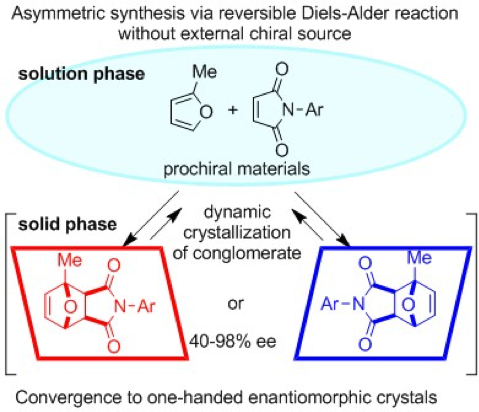
-
Crystallization-induced diastereomer transformation of thiohydantoin derivatives, Naohiro Uemura, Yasushi Yoshida, Takashi Mino, Masami Sakamoto, Tetrahedron, 2020, 76, 131166.
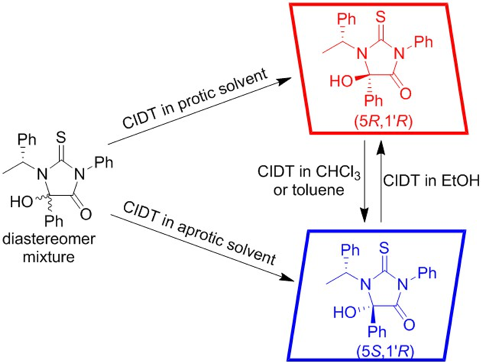
-
Visible-light-induced oxidative coupling reaction of benzylic aminesusing iridium(III) complex of pincer type imidazo[1,5-a]pyridine ligand, Fumitoshi Yagishita, Tatsuya Nagamori, Sota Shimokawa, Keita Hoshi, Yasushi Yoshida, Yasushi Imada, Yasuhiko Kawamura, Tetrahedron Lett., 2020, 61, 151782.
-
Attrition-Enhanced Deracemization of Axially Chiral Nicotinamides, Hiroki Ishikawa, Kazuma Ban, Naohiro Uemura, Yasushi Yoshida, Takashi Mino, Yoshio Kasashima, Masami Sakamoto, Eur. J. Org. Chem. 2020, 1001–1005.
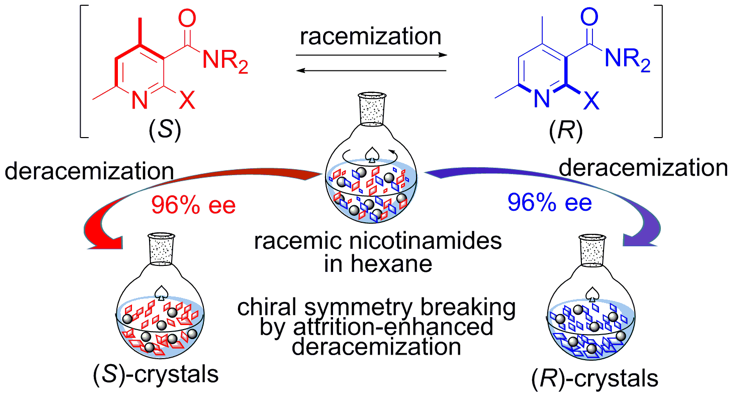
-
A new class of polychlorinated compounds derived from o-chloranil, Shiotsuki, Masashi; Saito, Shogo; Nagahata, Hiroki; Iwamura, Takeru; Uemura, Naohiro; Yoshida, Yasushi; Mino, Takashi, Tetrahedron Lett., 2020, 61, 152268.
-
A new class of dimeric product isolated from the fungus Chaetomium globosum: evaluation of chemical structure and biological activity, Sarmales-Murga, Christopher; Akaoka, Fumito; Sato, Michio; Takanishi, Jun; Mino, Takashi; Miyoshi, Noriyuki; Watanabe, Kenji, J. of Antibiot., 2020, 73, 320–323.
-
Regioselective Bay-Functionalization of Perylenes Toward Tailor-Made Synthesis of Acceptor Materials for Organic Photovoltaics, Keisuke Fujimoto, Seiichiro Izawa, Yusaku Arikai, Shinya Sugimoto, Hirona Oue, Toshiyasu Inuzuka, Naohiro Uemura, Masami Sakamoto, Masahiro Hiramoto, Masaki Takahashi, ChemPlusChem 2020, 85, 285–293.
-
Development of new catalytic enantioselective formation of methylenelactam-based N,O-spirocyclic compounds via ring opening-asymmetric reclosure of hydroxylactams, Tetsuya Sengoku, Ayako Miyoshi, Tamaki Tsuda, Toshiyasu Inuzuka, Masami Sakamoto, Masaki Takahashi, Hidemi Yoda, Tetrahedron, 2020, 76, 131252.
-
<Book>Asymmetric Synthesis Involving Dynamic Enantioselective Crystallization, Masami Sakamoto, Advances in Organic Crystal Chemistry, Comprehensive Reviews 2020, Eds. M. Sakamoto, H. Uekusa, Springer, 2020.


