|
|
|
|
|
|
|
|
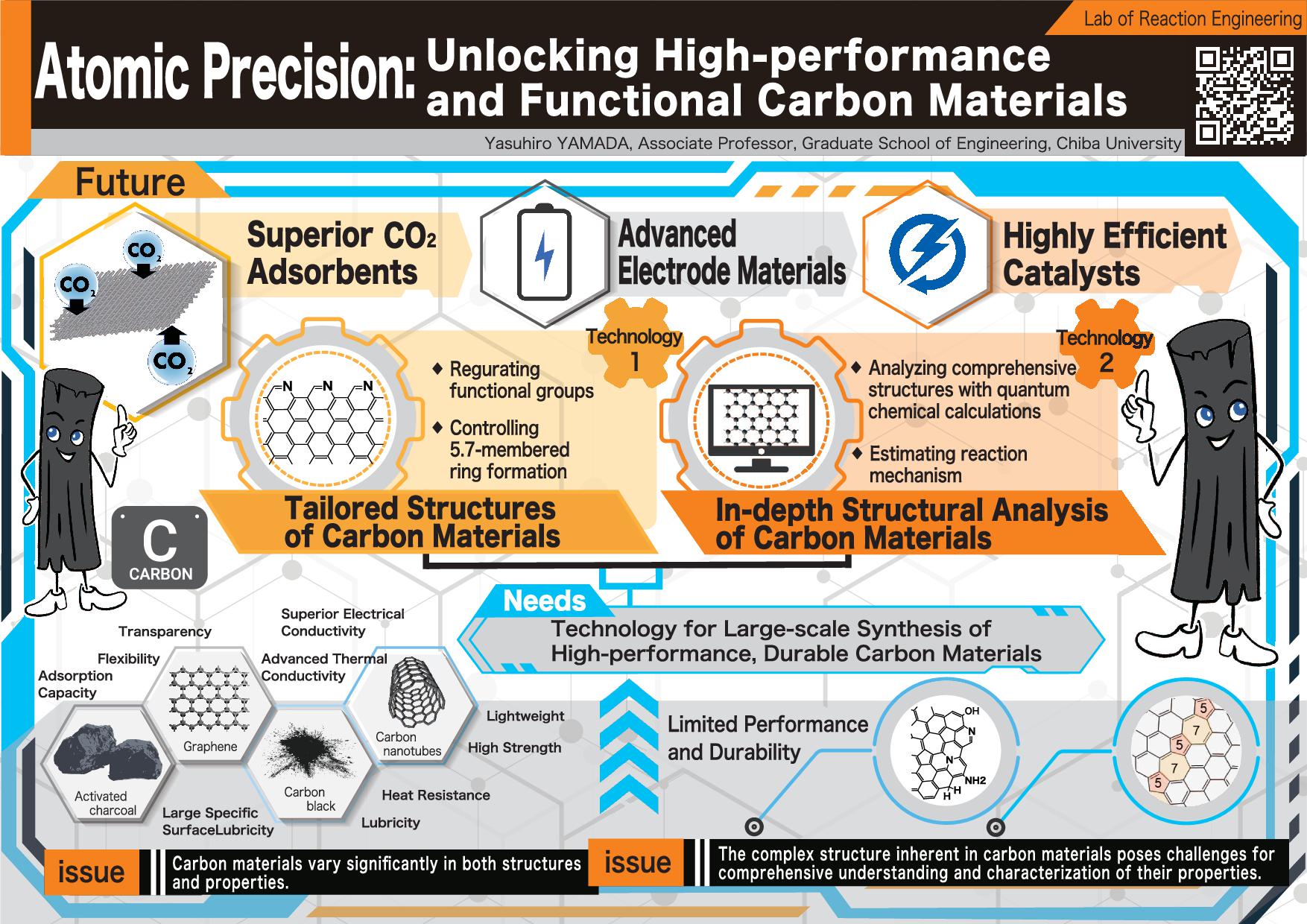
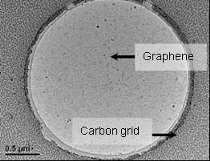
----------------------------------------------------------------------------------------- (1) Analyses of defect structures on graphene (1-1) Analyses by spectroscopy and microscopy Nano carbon materials include functional groups, vacancy defects, pentagons, and heptagons (Fig. 1-1). We analyze the defective structures of several-layered graphene using XPS, IR, TEM(Fig. 1-2), and Raman(Fig. 1-3).
Fig. 1-1 Types of defects on graphene Fig. 1-2 TEM image of graphene Fig. 1-3 Raman spectrum of graphene (1-2)Defective structure analysis using DFT calculation(XPS EIR): Various defects such as functional groups and point defects exist on edges and in the basal plane of carbon materials. Complete understanding of these defects is essential to maximize the properties of carbon materials. We have conducted simulation of XPS and IR spectra for oxygen-containing functional groups (Fig. 1-2-1)[1-2-1,1-2-2], nitrogen-containing functional groups [1-2-3], and other defects.
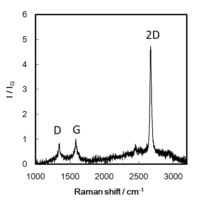
Fig.1-2-1 Graphene with OH groups [1-2-1] Fig.1-2-2 Simulated C1s XPS spectra of the left restructure [1-2-1] [1-2-1] Y. Yamada, et al., "Analysis of heat-treated graphite oxide by X-ray photoelectron spectroscopy", J Mater Sci 48 (2013) 8171-8198. [1-2-2] J. Kim, Y. Yamada, et al., "Pyrolysis of epoxidized fullerenes analyzed by spectroscopies", J Phys Chem C, accepted. [1-2-3] Y. Yamada, et al.,"Nitrogen-containing graphene analyzed by X-ray photoelectron spectroscopy", Carbon 70(2014)59-74. (2) Elimination of defects from nanocarbon materials Defects influence on the mechanical, electronic, thermal properties of carbon materials. Our goal is to heal defects in nano carbon materials. (3) Controlling structures of nano carbon materials Carbon materials contain the basal plane and edges. We introduced vacancy defects in the basal plane (Figs. 3-1, 3-2), and analyzed the defect structure [3-1]. Structures of carbon materials can be controlled by bottom-up approach without using catalysts (Fig. 3-3) [3-2, 3-3, 3-4]. 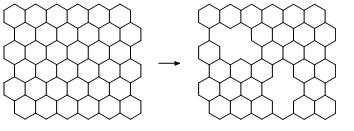
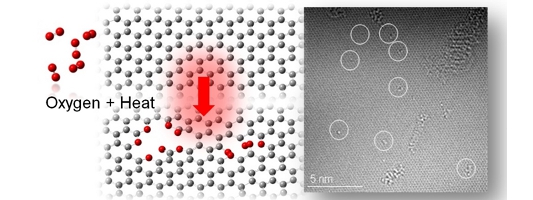
Fig.3-1 @Process to introduce vacancy defects
Fig.3-2 Sub-nanometer vacancy defects introduced on graphene by oxygen gas [3-1] Y. Yamada, K. Murota, et al., "Subnanometer vacancy defects introduced on graphene by oxygen gas", J Am Chem Soc 136(6)(2014)2232-2235. 
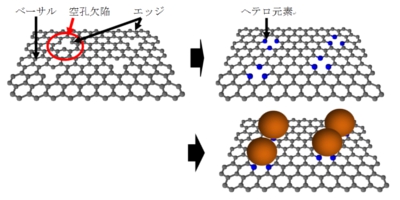
Fig.3-3 @Image to screen precursors using computational methods for structurally controlled carbon materials [3-2] Y. Yamada*, H. Sato, S. Gohda, T. Taguchi, S. Sato, Toward strategical bottom-up synthesis of carbon materials with exceptionally high basal-nitrogen content: development of screening techniques, Carbon 203 (2023) 498-522. https://doi.org/10.1016/j.carbon.2022.11.043 Available at SSRN:http://dx.doi.org/10.2139/ssrn.4210347 [3-3]Y. Yamada*, H. Tanaka, Y. Tanaka, S. Kubo, T. Taguchi, S. Sato, Toward strategical bottom-up synthesis of carbon materials with exceptionally high pyridinic-nitrogen content: development of screening techniques, Carbon 198 (2022) 411-434. https://doi.org/10.1016/j.carbon.2022.06.069 [3-4] R. Kawai, Y. Yamada*, S. Gohda, S. Sato, Bottom-up synthesis of carbon materials with high pyridinic-nitrogen content from dibenzacridine isomers with zigzag and armchair edges, J. Mater. Sci. 57 (2022) 7503-7530. https://doi.org/10.1007/s10853-022-07104-z (4) Graphene complex We have prepared metal ion coordinated graphene (Fig. 4), and analyzed the agglomeration mechanisms of coordinated metal ions on graphene [4-1, 4-2].
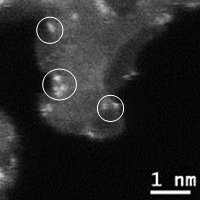
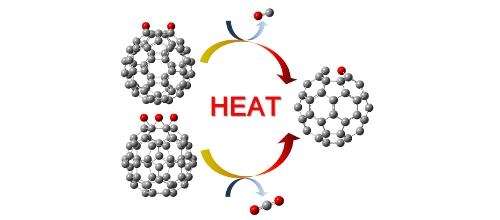
Fig. 4 @Introduction of hetero atoms at vacancy defects and coordination of metal ions and @STEM image of metal ion coordinated graphene [4-1] [4-1] Y. Yamada, M. Miyauchi, et al., "Exfoliated graphene ligands stabilizing copper cations", Carbon 49 (2011) 3375-3378. [4-2] Y. Yamada CY. Suzuki CH. Yasuda Cet al. C"Functionalized graphene sheets coordinating metal cations", Carbon 75 (2014) 81-94. (5) Dehydrogenation reaction of poly aromatic hydrocarbons We have prepared carbon materials by dehydrogenation of various poly aromatic hydrocarbons. (6) Clarification of mechanisms for migration of functional groups and their gasification Carbon materials contain functional groups in the basal plane and on edges. We have attempted to understand the mechanisms of migration of functional groups and their gasification upon heat treatment [6-1,6-2].
Fig. Pyrolysis of epoxidized fullerenes (Calculated by Gaussian03) [6-1] J. Kim, Y. Yamada*, et al., "Oxygen migration and selective CO and CO2 formation from epoxidized fullerenes", J Phys Chem C 118(13)(2014)7085-7093. [6-2] J. Kim, Y. Yamada*, et al., "Pyrolysis of epoxidized fullerenes analyzed by spectroscopies", J Phys Chem C 118(13)(2014)7076-7084. |